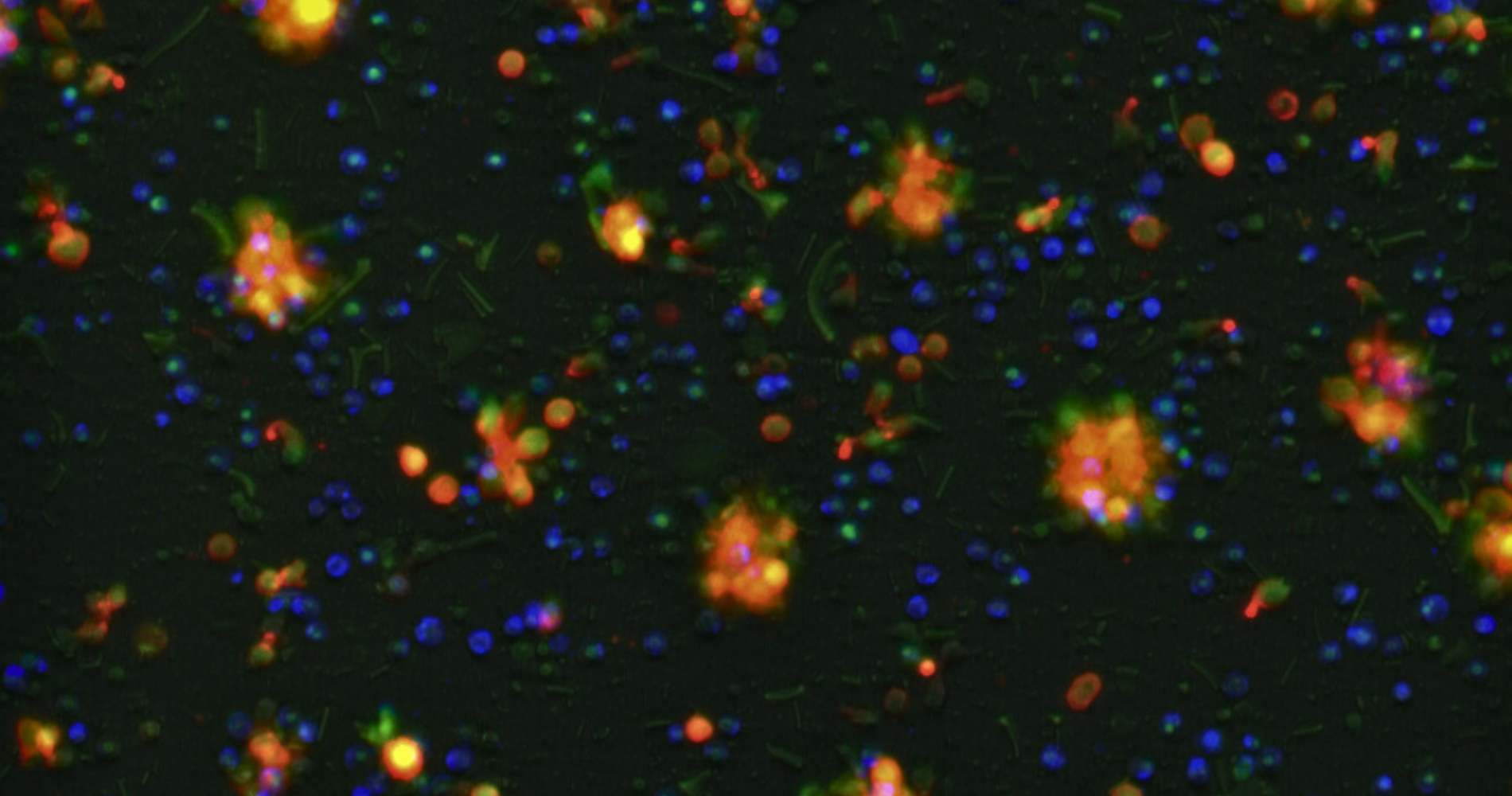
Welcome to Our Laboratory
Our primary research interest is to develop effective, safe, and off-the-shelf cancer immunotherapy. Adoptive immunotherapy is an especially promising therapeutic approach as represented by recent success of chimeric antigen receptor (CAR)-engineered T cell therapy against hematologic malignancies. Our overarching goal is to cure cancer by using the immune system.
Professor Yuki Kagoya, M.D., Ph.D.
News
- 2024.06.04 Research Associate newly joined.
- 2024.04.26 Our research was published in Cell Reports Medicine.
- 2024.04.01 Postdoc researchers and graduate students newly joined.
- 2023.11.20 Our research was published in Nucleic Acids Research.https://www.keio.ac.jp/ja/press-releases/2023/11/22/28-154650/
- 2023.08.17 Our review article was published in International Immunology.
- 2023.03.31 Our website has been opened.
- 2023.03.22 Our research was published in International Immunology.
- 2023.03.11 Our review article was published in Inflammation and Regeneration.
Latest news
- We have updated our website.
 by ailesamiWe have updated our website.
by ailesamiWe have updated our website.